Help Make Menu Labeling Work for Convenience Retailers – File Comments with FDA
Comments on FDA’s menu labeling final rule are due on August 2, 2017. It is critical that the Food and Drug Administration (FDA) hear from you. If you own or operate a chain of 20 or more convenience stores–or even a single franchise store if the franchise is part of a chain of 20 or more locations doing business under the same name–you are going to be responsible for implementing FDA’s rule. You also know how your store operates, the burdens the requirements would have on your business, and the ultimate effect it would have on the customers you serve.
FDA has publicly stated that it wants to hear directly from retailers on ways it might reduce the regulatory burden and increase flexibility around the disclosure of nutrition information to consumers. While NACS will be submitting comments on behalf of the industry, we cannot replicate the personal testimony each one of you can provide with your comment letters. Only you can accurately describe your business model, the marketing pieces in your store, and how they differ from menus. Only you can describe the buffets and grab-and-go food selection your store offers. Only you can tell FDA about the cost of and uncertainty surrounding menu-labeling compliance for your business.
Your comment letter will help FDA better understand the unique business models employed by convenience stores, the ways convenience stores differ from restaurants, and why FDA should rework its final rule to relieve the regulatory burdens the menu labeling rule will have on your business.
Learn more about the FDA’s existing onerous menu labeling rule.
1. Personalize the template letter NACS has prepared for you here .
- NACS has left you highlighted, bracketed places in the letter where you should fill in personal information about your company and your company’s experience with displaying nutrition information to customers.
- Remember to delete these brackets from the final letter!
- Feel free to further customize this letter. The more varied and personalized the letter, the more powerful it will be.
2. Save a copy of your letter to your computer.
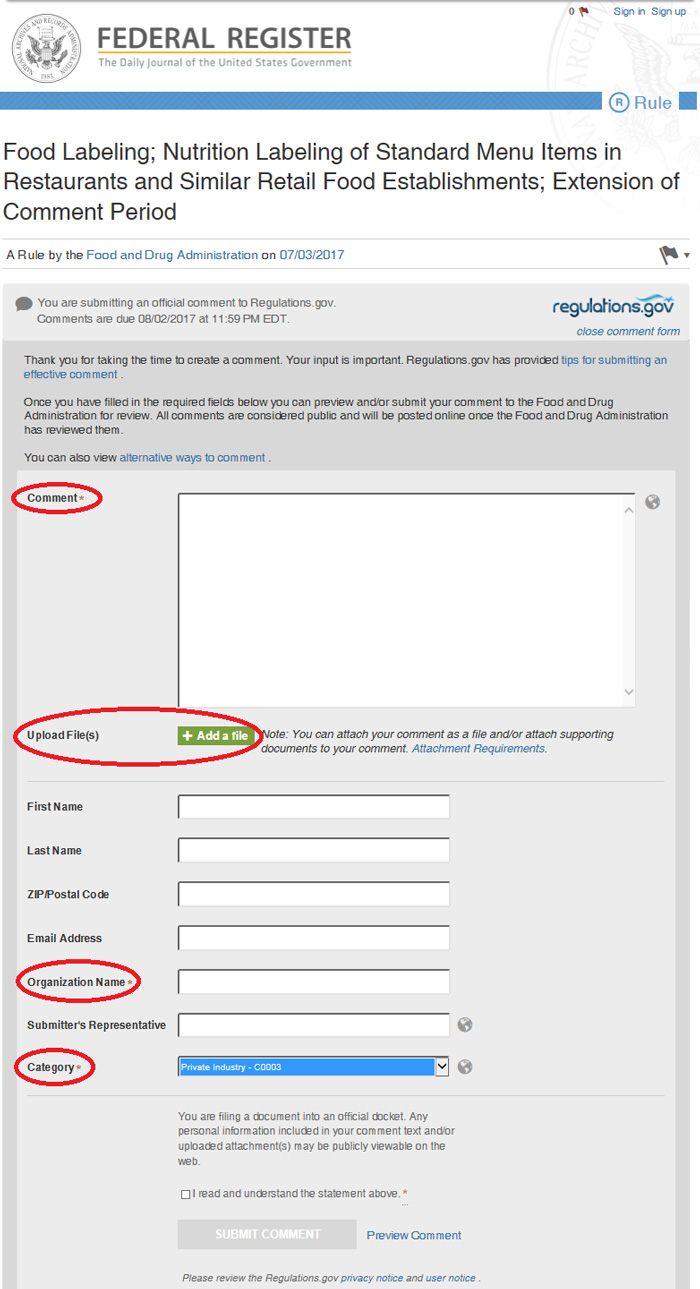
3. Upload your comments on this website.
- Fill in the requisite information:
- In the “Comment” field, type: “Please see attached file.”
- Under “Organization Name”, type your company name
- Under “Category,” select “Private Industry”
- Upload your saved letter(!)
- Click “continue” to advance to the next screen
- On the next screen, review all the information you have entered to ensure there are no mistakes
- Certify that you have read and understood the official statement
- Click “Submit Comment” to file your comments with the Food and Drug Administration!
* After clicking “submit” you will be prompted to put in an email address in order to receive a filing confirmation. We highly recommend you do this although it is not necessary
4. Please email a copy of your comments to Jon Taets, NACS Director of Government Relations: jtaets@convenience.org